
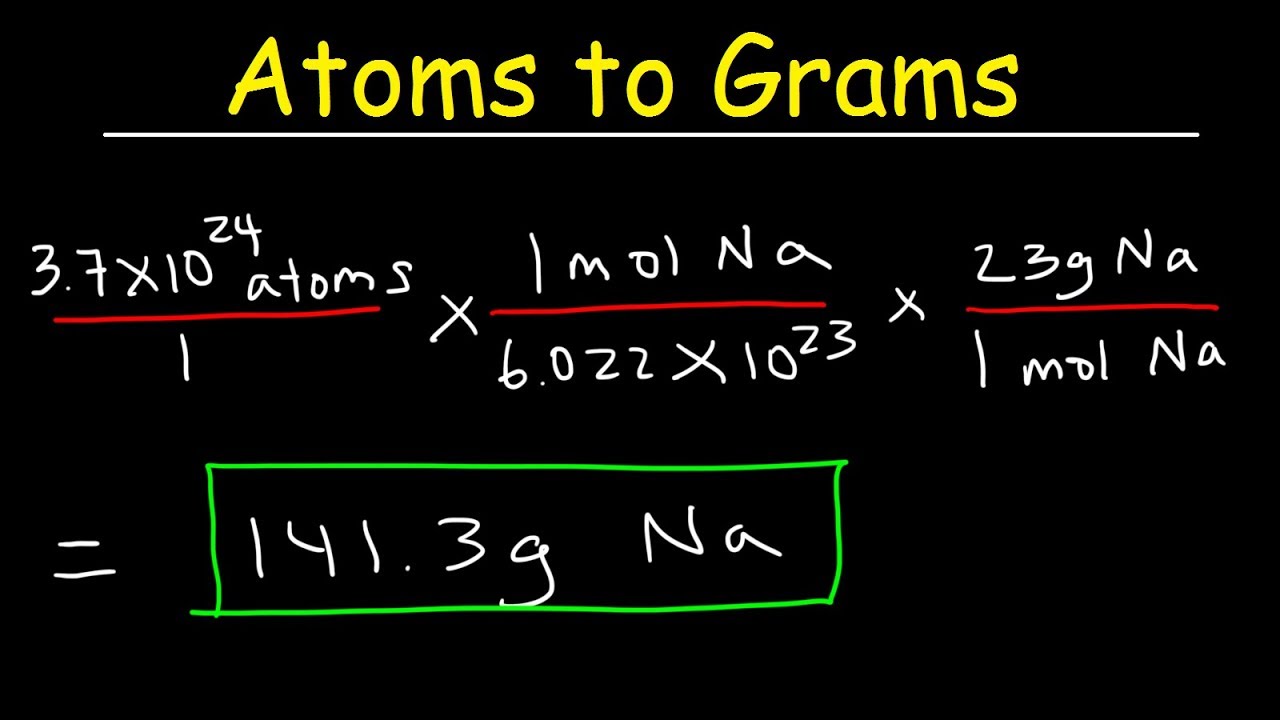
Multiply them by the molar mass constant, and then multiply the result by 2.

This means that if you want to find the molar mass of elements that are composed of 2 atoms, such as hydrogen, oxygen, and chlorine, then you'll have to find their relative atomic masses.
Some elements are only found in molecules of 2 atoms or more. This converts atomic units to grams per mole, making the molar mass of hydrogen 1.007 grams per mole, of carbon 12.0107 grams per mole, of oxygen 15.9994 grams per mole, and of chlorine 35.453 grams per mole. This is defined as 0.001 kilogram per mole, or 1 gram per mole. Multiply the relative atomic mass by the molar mass constant.






 0 kommentar(er)
0 kommentar(er)
